
A Message from the Director
Happy New Year, all!


First, I would like to take a moment to thank all clients and stakeholders for your continued support of the VMDL.
Looking back, we had a challenging and yet successful 2023. The VMDL processed approximately 64,924 accessions and performed more than 207,026 laboratory tests, including 47,262 tests for foreign animal disease and public health surveillance. The diagnostic service was utilized by veterinarians, owners, and researchers from 110 Missouri counties and independent cities and 28 other states.
In addition, the VMDL retained full accreditation by AAVLD, NAHLN Level 1 and FDA Vet-LRN Tier 1 lab status, NPIP certification, and USDA EIA Lab certification. The VMDL One Health – Rabies Lab was established and began testing pets’ samples for rabies vaccination status.
The faculty taught (or co-taught) 28 DVM, graduate, and undergraduate courses and 20 clinical or diagnostic rounds. The VMDL worked on 19 funded research projects, published 26 peer-reviewed papers, and presented 25 conference posters and oral presentations. The faculty and staff also gave 29 extension and outreach presentations. VMDL faculty also provided leadership services to many international, national, state and local organizations.
VMDL Phase 1 construction will be completed in the fall. The rest of the VMDL facility needs will be addressed by the recent state funding ($43 million). Thank you from the bottom of my heart!
Shuping Zhang, Professor and Director of VMDL
Johne’s Disease — Not Just for Dairy Cows
By Rosalie Ierardi, DVM, MS, Clinical Instructor

Despite its reputation as a disease of dairy cattle, Johne’s is a significant concern for beef producers as well. In a 2014 survey, 27 percent of U.S. beef producers reported at least one clinically affected animal during the previous year. Cow-level prevalence in cow-calf herds ranges from 1.5 percent to 3 percent in the United States and Canada, with between 10 percent and 24 percent of herds having at least one positive animal.
Seedstock producers are more likely to enroll in voluntary control programs for Johne’s disease and test their herds. They also tend to have a lower prevalence of infected animals. Seedstock producers also stand to lose the most financially if Johne’s is introduced, as this directly impacts their ability to market breeding stock and can result in the loss of valuable genetics.
It is difficult to measure the economic impact of Johne’s disease in beef herds. However, in one study of more than 4,800 cows from three cow-calf herds, the 205-day adjusted weaning weight (AWW) of calves from cows with a positive antibody test was 47.3 pounds less than calves from cows with a negative antibody test. The AWW of calves from “heavy shedders” (cows with a strongly positive Johne’s fecal culture) was 128.7 pounds less than calves from culture-negative cows. Based on 2007-2011 feeder calf prices, estimated losses were $57/calf for cows with a positive antibody test and $157/calf for cows with a strongly positive fecal culture.
Calves are typically exposed via manure from infected adult cattle. The Johne’s bacterium can survive for months in soil, manure, and contaminated water. Allowing mature cows to access calving areas has been shown to increase the risk of transmission to calves. Introduction of newly purchased animals is also associated with increased risk; however, fewer than 2 percent of beef producers test new cattle for Johne’s.
Common tests for Johne’s disease are fecal culture, fecal PCR, and a blood antibody test (ELISA). Fecal culture is considered the gold standard; however, culture takes six to seven weeks. Johne’s culture also requires specialized equipment and growth media, so many labs, including the VMDL, do not run this test. Fecal PCR is another option with results generally available within two weeks. Fecal PCR is not as sensitive as culture. However, this disadvantage is usually offset by its much faster turnaround time.
ELISA testing is much less sensitive for Johne’s, but it is more affordable and can be performed quickly on many animals. ELISA is effective for annual screening at the herd level, but is less useful for individual animals, particularly young stock.
Annual whole herd testing with PCR on individual cattle is an alternative that circumvents the low sensitivity of ELISA but is too expensive to be practical for most producers. Whole herd testing with individual PCR every two years offered similar results at lower cost, as did testing the whole herd with pooled PCR (five samples/pool) annually. Risk-based sampling is also an option for some herds, which involves testing only a subset of animals.
Keeping Johne’s disease out of the herd in the first place is best, as minimizing its impact in herds that are already infected is more difficult. Total eradication is probably not cost-effective for most commercial cattle producers. However, prompt removal of shedders and clinical cattle helps to minimize losses. Tradeoffs between test performance, cost, and turnaround time make test selection a unique decision for every herd.
Canine Respiratory Disease – What Have We Found?
By Zhenyu Shen, BVM, MS, PhD, Assistant Research Professor

Recently, an atypical canine infectious respiratory disease has caught the attention of many veterinarians and owners. Over the past two months, the VMDL has worked on some suspect cases. In addition to routine bacteriology and PCR tests, we performed deep sequencing on the respiratory samples. We use a method called RNA sequencing, which catches any messenger RNA/mRNA, a type of genetic material that is produced by living organisms. So far, the deep sequencing detected the presence of many commensal or opportunistic pathogens, such as Acinetobacter spp., Frederiksenia canicola, Moraxella canis, Mycoplasma cynos, Pasteurella multocida, Pseudomonas spp., Staphylococcus spp., and Streptococcus spp. However, we have not found any common microbes or viruses that may contribute to the disease. The sequencing work is supported by a USDA grant and is free to clients. We will keep working on the samples submitted to us and seek collaborations with researchers at MU and other universities. Hopefully, the causative agent will be identified soon.
FAVN Testing: Where are the Samples Coming From and Where are the Pets Going?
The MU VMDL is proud to offer rabies serology by the WOAH FAVN method for pet travel. The following graphic depicts the location of veterinary clinics submitting for FAVN testing by state. Thank you for your support!
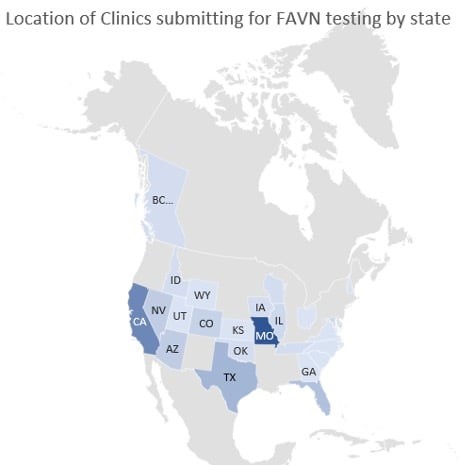
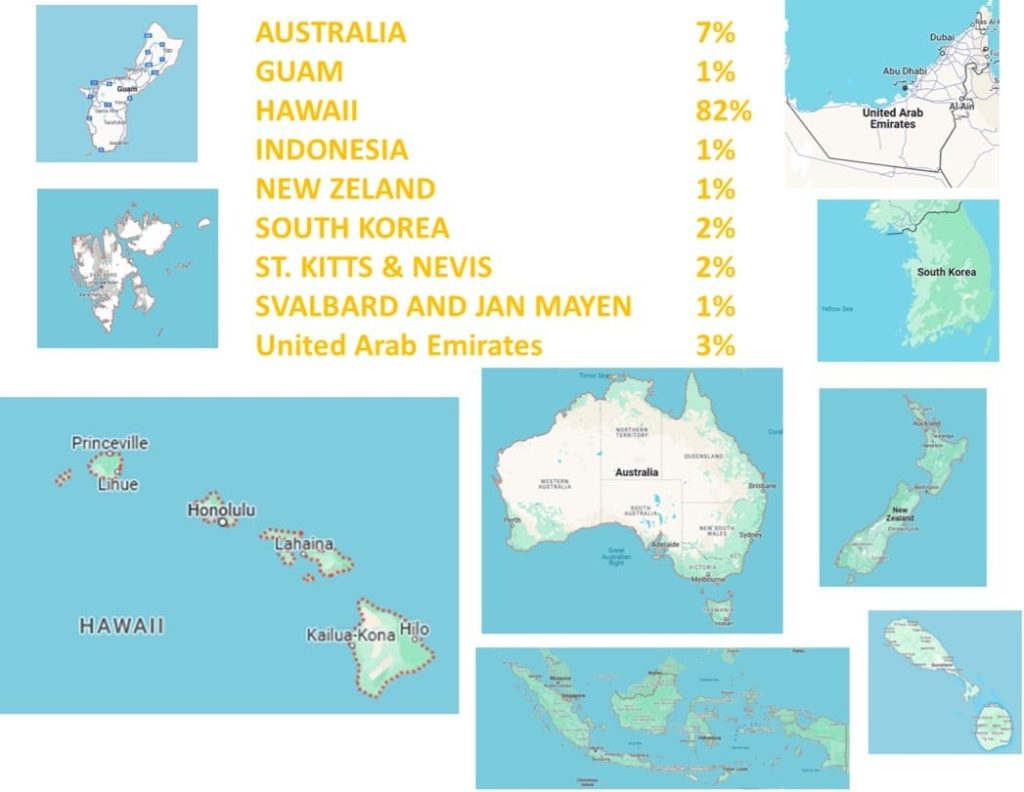
The following figure shows the travel destinations of pets requiring FAVN testing through the MU VMDL. While Hawaii is the most popular destination, pets are traveling all over the world!
UNDER THE SCOPE – WINTER 2024
