
New VMDL Fee Guide Available! Pricing Effective July 1

Histopathology/Biopsy Changes:
- Our base biopsy price ($72.50) will now include evaluation of up to three sites from the same animal. Additional sites from the same animal will be charged an additional $30 per site.
- A dissection fee ($30) will be assessed for large surgical biopsies such as amputated limbs, whole spleens, mandible/maxilla specimens, whole mammary chains, digits, and very large masses. These cases require a significant amount of additional pathologist time to locate the lesion(s) and collect appropriate sections.
- A new test code has been created for formalin fixed tissues derived from an offsite necropsy examination. This test is called “Fixed Tissue Exam- from necropsy” and there is no limit on the number of fixed tissues/organs that can be submitted. The cost for this evaluation is $100 and may not be used for tissue excised from a live animal during a surgical procedure.
Disposal Changes (with necropsy only):
- Disposal/Incineration fees now apply for communal cremation of avian and companion animals, as well as food animals weighing 500 pounds or less. Please see page 4 for details.
Tests Added:
- Equine Neonatal/Foal Enteric PCR Panel: Rotavirus A PCR, Salmonella PCR, C. difficile toxin A&B and C. perfringes toxin ELISA ($90)
Tests Discontinued:
- Feline Heartworm Antibody Lateral Flow Immunoassay
- Equine Viral Arteritis ELISA
If you have any questions about our new fee guide, please feel free to call the laboratory at 573-882-6811. We strive to provide high quality diagnostics at a reasonable price.
Bone Biopsy: Sampling for Success
 Using histopathology to definitively diagnose canine bone lesions is very important, since benign and neoplastic osteodestructive processes can be indistinguishable on radiographs and diagnosis greatly affects the treatment regimen. However, representative sampling of the lesion by bone biopsies (e.g., Jamshidi bone needle, Michele trephine) can be quite challenging. The center of the lesion is often necrotic, while the edges may simply be reactive changes of bone and periosteum. Sometimes, despite everyone’s best efforts, the tissue sampled is non-diagnostic. A retrospective study of VMDL cases demonstrates that neoplastic changes were not observed in 20 out of 61 (33 percent) primary bone tumor cases in dogs at the first bone-core biopsy attempt (Kuroki et al. Accuracy of bone-core biopsy for diagnosis of primary bone tumors in dogs. 2012 ACVP and ASVCP annual meeting. Paper #D-47). While it’s a good idea to prepare your clients for the possibility of an unsuccessful diagnosis, here are a few things you can do to improve your chances of getting a definitive diagnosis:
Using histopathology to definitively diagnose canine bone lesions is very important, since benign and neoplastic osteodestructive processes can be indistinguishable on radiographs and diagnosis greatly affects the treatment regimen. However, representative sampling of the lesion by bone biopsies (e.g., Jamshidi bone needle, Michele trephine) can be quite challenging. The center of the lesion is often necrotic, while the edges may simply be reactive changes of bone and periosteum. Sometimes, despite everyone’s best efforts, the tissue sampled is non-diagnostic. A retrospective study of VMDL cases demonstrates that neoplastic changes were not observed in 20 out of 61 (33 percent) primary bone tumor cases in dogs at the first bone-core biopsy attempt (Kuroki et al. Accuracy of bone-core biopsy for diagnosis of primary bone tumors in dogs. 2012 ACVP and ASVCP annual meeting. Paper #D-47). While it’s a good idea to prepare your clients for the possibility of an unsuccessful diagnosis, here are a few things you can do to improve your chances of getting a definitive diagnosis:
- Sample multiple areas of the lesion! In our retrospective study one or two biopsy sample(s) yielded approximately 60 percent positive diagnosis while three or more biopsy samples yielded 93 percent positive diagnosis. If possible, it’s best to sample the radiographic center of the lesion as well as areas of sclerosis on both sides.
- Use imaging to plan and confirm biopsy sites.
- Provide a detailed history with the specimens, ideally including an image or diagram showing the number and location of the biopsy sites.
Possible complications associated with bone-core biopsies include bone fracture, nerve or blood vessel damage and infection. If an amputation is performed, clinicians may submit the entire fresh limb to the VMDL for adequate sampling and histopathology examination. Please feel free to call the laboratory with questions regarding bone biopsy or whole limb submission.
Bacillary Hemoglobinuria in Missouri
By: Rosalie Ierardi, DVM
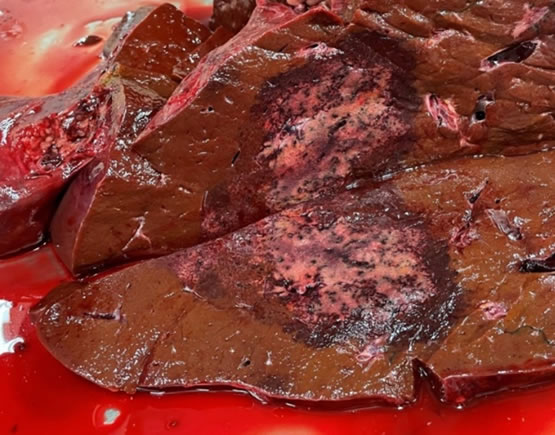
By now, many of you have heard about a recent cluster of bacillary hemoglobinuria cases in north-central Missouri. At least seven cattle between December 2020 and May 2021 met the criteria of hemoglobinuria, hepatic necrosis, and identification of Clostridium novyi/Clostridium haemolyticum in the liver lesions. In five of these cases, deer flukes and/or fluke eggs were identified in the liver. Seven cattle may not sound like much, until one considers that during the previous decade (2010-2020) only three suspected cases of clostridial hepatitis were received by the VMDL. We are continuing to investigate this group of cases, and diagnostic submissions are highly encouraged.
In any discussion of bacillary hemoglobinuria, it’s worthwhile to consider two closely related conditions with a very similar pathogenesis: bacillary hemoglobinuria, “red water disease” versus infectious necrotic hepatitis, “black disease”.
Bacillary hemoglobinuria (BH) is caused by Clostridium haemolyticum, which produces a toxin that causes intravascular hemolysis and acute hepatic necrosis. The hemolysis causes severe anemia, and the disease is almost invariably fatal.
Sudden death is frequent. Clinical signs include depression, anorexia and fever. Hemoglobinuria is the hallmark. At necropsy, the most consistent and characteristic lesion is a focally extensive area of hepatic necrosis, often surrounded by a dark red halo.
Infectious necrotic hepatitis (INH) is a similar condition which occurs commonly in sheep, occasionally in cattle, and rarely in horses. INH is caused by Clostridium novyi type B. Liver lesions are fairly similar to those in BH, but hemoglobinuria is absent. Like BH, this disease is nearly always fatal.
Both C. haemolyticum and C. novyi type B live in soil as highly resistant spores. Once ingested by grazing animals, spores can lay dormant in the liver for months. Disease occurs only when liver injury creates the anaerobic conditions required for bacterial growth. This is typically attributed to migration of Fasciola hepatica (common liver fluke), but bacillary hemoglobinuria is also reported in cattle without evidence of liver flukes. While Fasciola hepatica is thought to be the most common predisposing factor for BH, this species is seldom reported in Missouri. Missouri cattle are more likely to be infected with Fascioloides magna, the deer fluke. The relationship between F. magna and BH is less thoroughly studied.
F. hepatica and F. magna have similar and intricate life cycles. Development of the flukes depends on availability of snail intermediate hosts, a moist environment, and temperatures above 50 degrees Fahrenheit. Fluke eggs are deposited in the feces of cattle and sheep (F. hepatica) or deer (F. magna). Eggs hatch and develop through several life stages, mostly within snails, until they mature into the infective larval stage known as metacercariae. Metacercariae encyst on vegetation, awaiting ingestion by the mammalian host. In favorable conditions, eggs and/or metacercariae can survive on pastures for months.
Once ingested, F. hepatica migrates to the liver and eventually settles in the bile ducts, where its eggs are passed into the feces. In the case of F. magna, cattle and sheep are dead-end hosts. The flukes wander aimlessly in the liver and cause considerable damage until they are walled off with fibrous connective tissue by the host. The eggs never make it into the feces.
When examining a bovine carcass with hemoglobinuria and/or icterus, examine the liver carefully. Necrotic lesions are sometimes subtle. Migration tracts of F. magna within the liver typically contain black pigment. It’s best to submit multiple small formalin-fixed samples for histopathology and several large fresh samples for bacterial culture. Laboratory confirmation can be challenging, as C. haemolyticum and C. novyi type B are difficult to isolate. Diagnosis is supported by fluorescent antibody testing (FAT), which is routinely performed at the VMDL. Ideally, carcasses should be burned, buried, or removed from the premises to reduce contamination of soil.
Vaccination is the best way to protect cattle from BH and INH. To prevent bacillary hemoglobinuria, the vaccine must be labeled for protection against C. haemolyticum. Most “seven-way” multivalent vaccines do not protect against BH, but C. haemolyticum is usually included in eight-way or nine-way products. Duration of immunity is only about six months; in especially high-risk situations, biannual vaccination may be warranted. Although avoidance of liver flukes is an attractive target for disease control, this is often an impractical approach.
