
Meet Dr. Susan Moore
Meet Susan Moore, PhD. Dr. Moore is a rabies expert and certified Clinical Laboratory Improvement Amendments director. Please join us in welcoming her as she works to set up the One Health Laboratory at the VMDL.
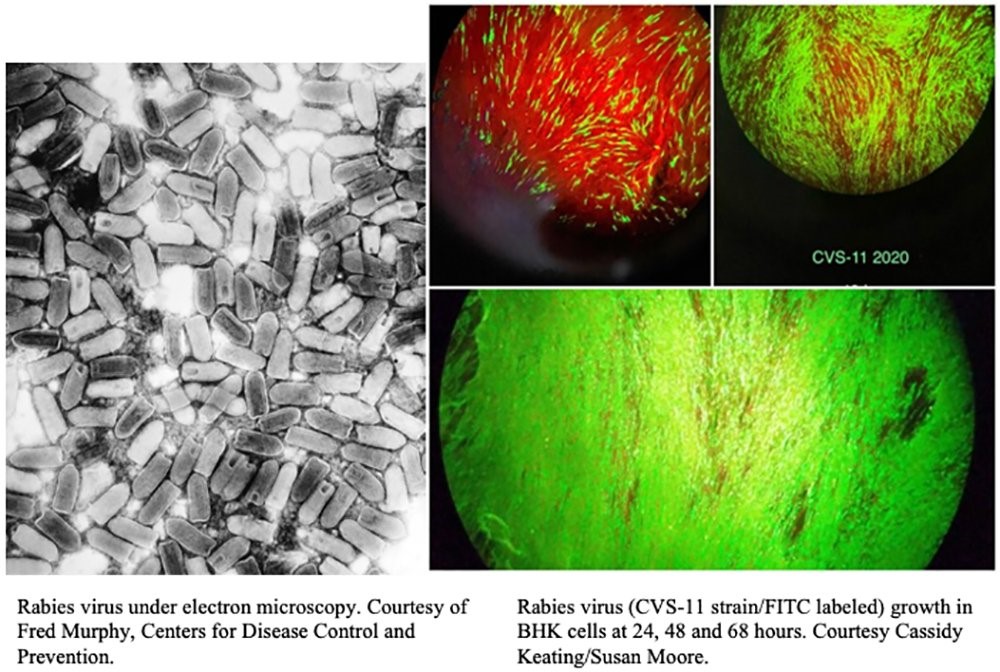
After a first career in human clinical laboratory science, specializing in blood banking, I switched to rabies laboratory work in 1999. While continuing to work in the rabies field, I earned my MS and PhD degrees in pathobiology at Kansas State University. I am a certified High Complexity Laboratory director through the American Association of Bioanalysts, which allows me to serve as a CLIA director overseeing human clinical laboratory testing. I was the director of the KSU Rabies Laboratory, one of the largest rabies serology laboratories in the world overseeing both animal and human testing for diverse purposes such as pet export and clinical trials, working in rabies serology for 21 years. I serve on various advisory committees and work groups for World Health Organization, U.S. Department of Agriculture, and Centers for Disease Control and Prevention, among others. I have published extensively and given presentations on rabies, serology, vaccines, and control and prevention measures; rabies prevention is an excellent example of a One Health approach.
The need for specialized rabies serology and diagnostic services continues to grow. Prevention of importing rabies into rabies-controlled countries by requiring proof of vaccination through rabies serology and defining the breadth of specificity of new rabies monoclonal products for use in rabies post-exposure prophylaxis are just two examples of the diverse application of rabies serology testing. Both are being used in the challenge to end human rabies deaths by 2030, a concerted plan by world health partners, WHO-World Organisation for Animal Health-Food and Agriculture Organization-Global Alliance for Rabies Control, launched in 2015. Beyond these global efforts, concerns about vaccine reactions in dogs and cats have led to an increased use of rabies serology for booster vaccination decisions. WHO and Advisory Committee on Immunization Practices also recommend routine rabies vaccine titer checks for those in occupations (or with hobbies) that bring increased risk of rabies exposure. Supplying prompt and high-quality rabies serology results is my passion.
Once set up the One Health Laboratory at the University of Missouri Veterinary Medical Diagnostic Laboratory will offer rabies serology testing on animal samples, and later human samples, as well as become a broad service laboratory.
Winter Sample Shipping Tips
When the snow flies, you still need your samples to arrive safely at the VMDL. Here are a few common questions we get about shipping this time of year.
It’s so cold, can I skip the ice pack?
Remember that sorting facilities and delivery trucks are often heated despite the frigid outdoor temperatures. Throw in an ice pack (or two) and double up on the insulation to keep your sample in the refrigerated temperature range.
How do I protect formalin-fixed samples from freezing?
Adding alcohol is the easiest way to lower the freezing point of the fixative solution. The most readily available in the average veterinary practice is isopropyl alcohol (freezing point -128° F). You’ll need 10 percent of your total liquid volume to be alcohol to keep the jar from freezing. If you’re using 70 percent isopropyl alcohol, increase that to 15 percent.
They’re forecasting a huge storm this week; will you guys be there to receive samples?
The VMDL typically remains open to receive, process, and store samples despite campus closures. However, winter weather often has severe impacts on courier services (FedEx, UPS, etc.), particularly if it’s a large storm system. Despite our best efforts, your sample may be delayed in transit. Please plan ahead and communicate with us if you know your sample has been delayed. In some cases, it may be best to cancel the testing and resubmit if it has been in transit too long.
Looking for Information on Deer Flukes?
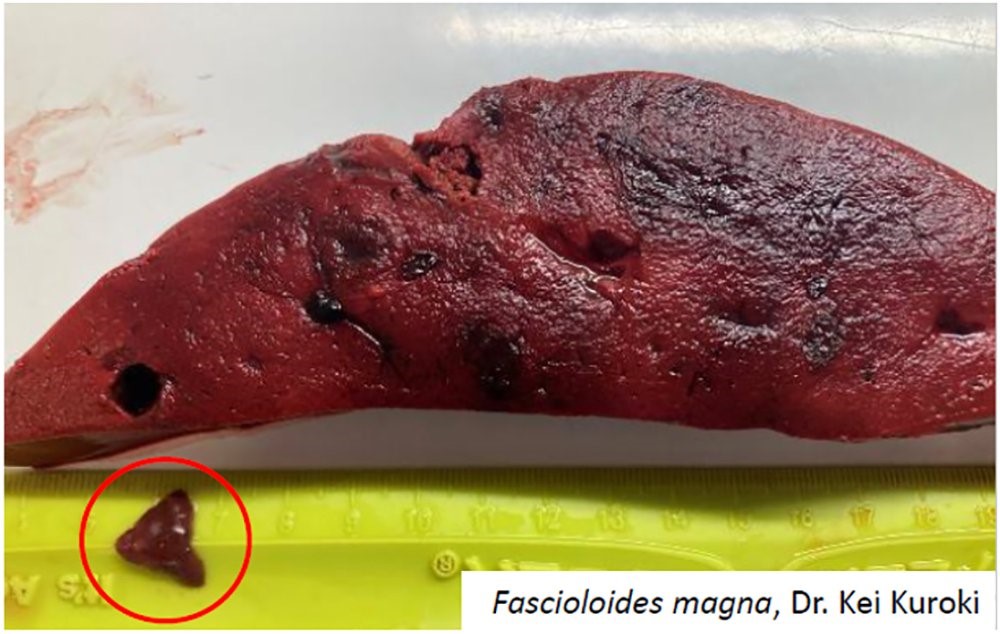
A new brief on the topic of liver flukes in Missouri is available through MU Extension, and can be found on their website here: https://extension.missouri.edu/publications/g2119
The MU VMDL can assist with diagnosis of liver flukes and associated conditions such as bacillary hemoglobinuria and infectious necrotic hepatitis. We offer fluorescent antibody testing for Clostridium spp., as well as anaerobic culture and complete necropsy services.
Biosecurity Resources for Pork Producer

There are many aspects to good biosecurity. As a NAHLN Level 1 Lab, the VMDL provides accurate and timely testing for important diseases of swine, and participates in active surveillance efforts for ASF/CSF.
Developing a Secure Pork Supply Plan for each premises is another important way to safeguard herds in the event of a disease outbreak. Producers can receive expert help with their Secure Pork Supply Plans by filling out the following questionnaire on the Rapid Access Biosecurity app: https://forms.gle/j1GP4ct8GCh16sfy5. More information on Secure Pork Supply Plans can be found at https://www.securepork.org/
